Abstract
Background: Cancer is associated with hypercoagulable state and frequently complicated by venous thromboembolism (VTE). Microparticles (MPs) are implicated in the pathogenesis of cancer associated thrombosis. However, not much is known about their interaction with the endothelium.
Aim: To study the effect of cancer cell derived MPs on the hemostatic balance of endothelial cells.
Methods: Microparticles released in conditioned medium from pancreas adenocarcinoma cells (BXPC3) and human breast cancer cells (MCF7) were isolated by differential centrifugation from cell cultures at 90% confluence. The pellets were washed twice in phosphate-buffered saline (BSA) and re-suspended in 1 ml Endothelial cell Basal Medium (EBM-2). Human umbilical vein endothelial cells (HUVEC) were cultured in 96-well plates in presence or absence of MPs derived from BXPC3 or MCF7 cells for 72h (C0). Then they were washed twice in BSA. and re-suspended in serum RPMI-1640 medium and re-cultivated over three cycles (C1-C3) following the same procedure of washing and culture.
Thrombin generation (TG) of normal platelet poor plasma (PPP) added in wells carrying HUVEC were assessed by the Calibrated Automated Thrombogram (Rousseau et al Thromb Res. 2015;136:1273-9). Furthermore, TF activity (TFa) was assessed with a clotting based assay (from Diagnostica Stago, France). Cells and MPs were analyzed by flow cytometry for TF and annexin V expression using specific antibodies.
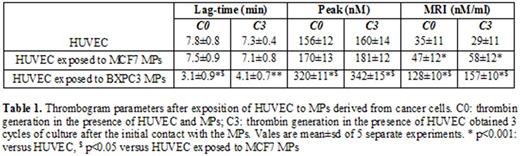
Results: Some traces of TFa were found in HUVEC cells (0.20±0.03pM) and HUVEC MPs (1.2±1.0pM) being comparable to normal PPP (0.19±0.02pM). Abundant amounts of TFa were found in BXPC3 cells (2.6±0.05pM) and BXPC3 MPs (920±50pM). MCF7 cells expressed low amounts of TFa (1.21±0.05pM) whereas TFa levels in MCF7 MPs were significantly higher (12.1±2.2pM). Flow cytometry analysis for TF expression showed similar results for the three types of cells and the corresponding MPs. No procoagulant phospholipids were found on the surface of the three cell lines. In contrast, procoagulant phospholipids were expressed by BXPC3 and MCF7 MPs but not by HUVEC derived MPs. HUVEC exposed to cancer cell derived MPs enhanced thrombin generation. This procoagulant property of HUVEC was acquired after exposure to MPs and remained significant after three cycles of cells culture. MPs derived from BXPC3 had more pronounced effect on HUVEC as compared to MPs derived from MCF7 (Table 1).
Conclusion:
Cancer cell-derived microparticles possess significantly higher procoagulant activity as compared to the cells of origin. They are characterized by high expression of TF and procoagulant phospholipids. Cancer cell-derived MPs induced shift of HUVEC hemostatic balance. Quiescent HUVEC were transformed to potent procoagulant cells and enhanced thrombin generation. The procoagulant properties acquired by HUVEC are transferred to the following cells generations. To the best of our knowledge, the present study shows for the first time that the inherent procoagulant properties of MPs could alter vascular functions. The impact of MPs on the procoagulant phenotype of the HUVEC varies according to the type of cancer cell line. Overall, the findings of the present study provide novel information regarding the specific contribution of tumor-derived MPs to the pathogenesis of cancer-associated thrombosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal